Krf2 Hybridization Of Central Atom
KrF2 Kr NH2Cl N CH2Br2 C SCN-1 C Could someone please show me how this is done. 3 Chemical and Physical Properties Expand this section.

Theories Of Covalent Bonding Ppt Download
Hybridization is a process where two or more than two atomic orbitals are mixed and formed new hybrid orbitals.
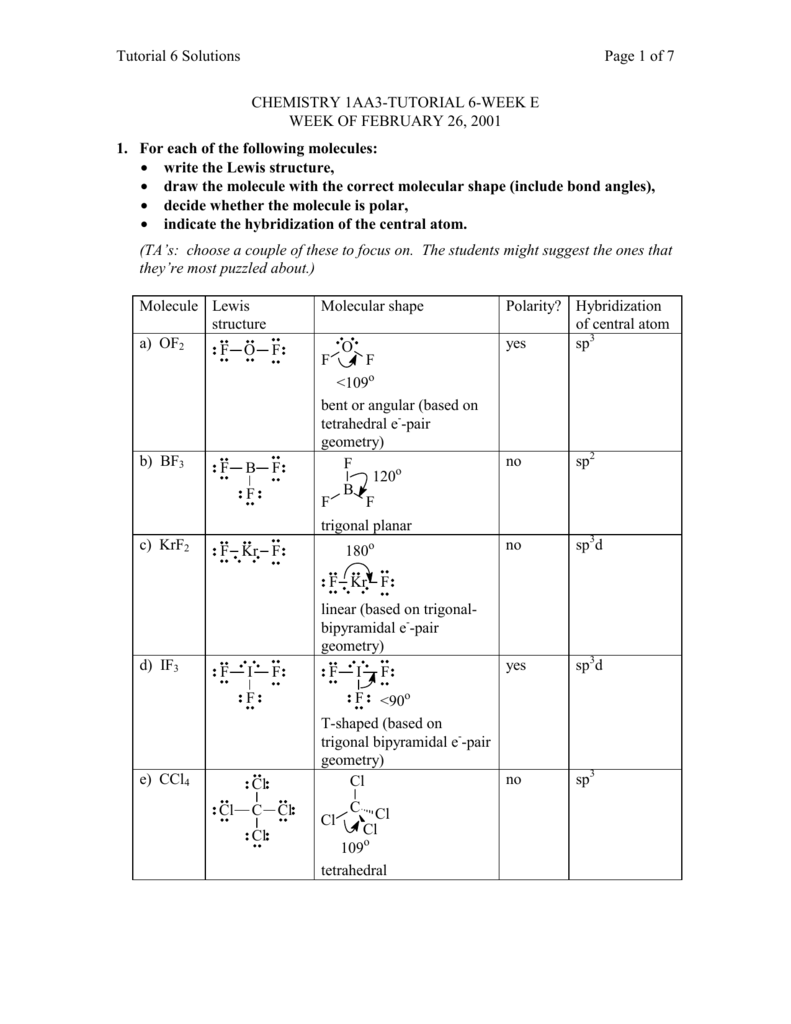
Krf2 hybridization of central atom. Is KrF2 linear. The stabilities of the dihalides follow the order KrF2 XeF2 RnF2 and XeF2 XeCl2 XeBr2. KRF2 XEOF4 03 BI3 Give the hybridization of the central atom parenthesis in.
The lone pairs will arrange themselves at 180 to each other. As F is univalent the number of sigma bonds in ClF5 is 5. Total bonds e-pairs.
Shape is determined by the relative placement of the bonded atoms around the central atom. Cl contains 7 valence electrons out of which 5 are involved in sigma bond formation with 5 Cl atoms and remaining 2 remains as a lone electron pair. The electron-domain geometry around this central atom is _____.
The calculated trends account for the fact that only the heavier noble gases form compounds. Although you would expect Krypton to have an electronegativity of zero as it is a noble gas when Krypton interacts with highly electronegative atoms like Fluorine it will essentially give up one of its electrons inducing a charge. CaCl Focus On Only One Of The Carbons Electron Lewis Structure Geometry VSEPR Sketch Hybridization Of Central Atom Formal Charges C C.
What is the molecular shape of KrF2 molecule and What is the molecular shape of KrF2. CO 2 2. 2 Names and Identifiers Expand this section.
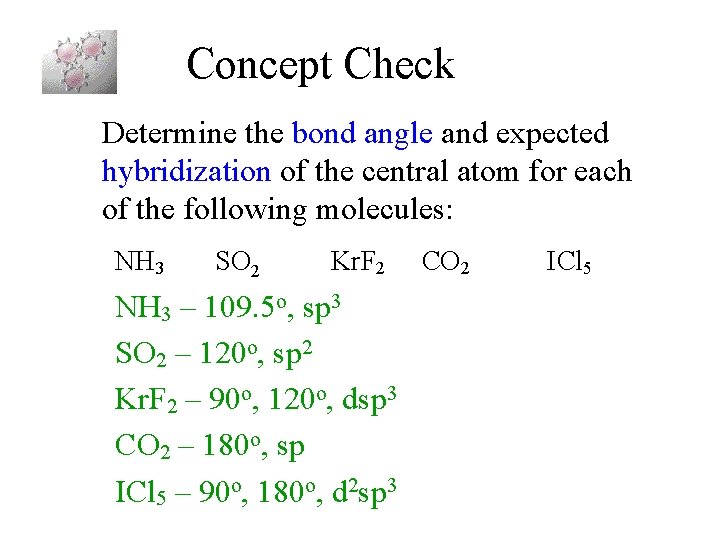
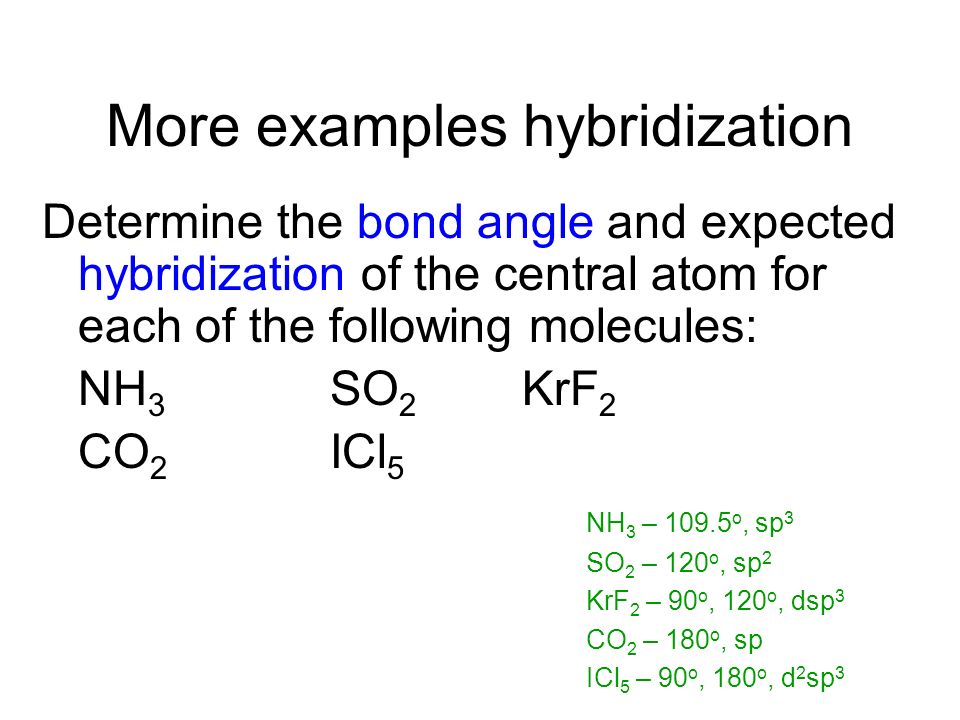
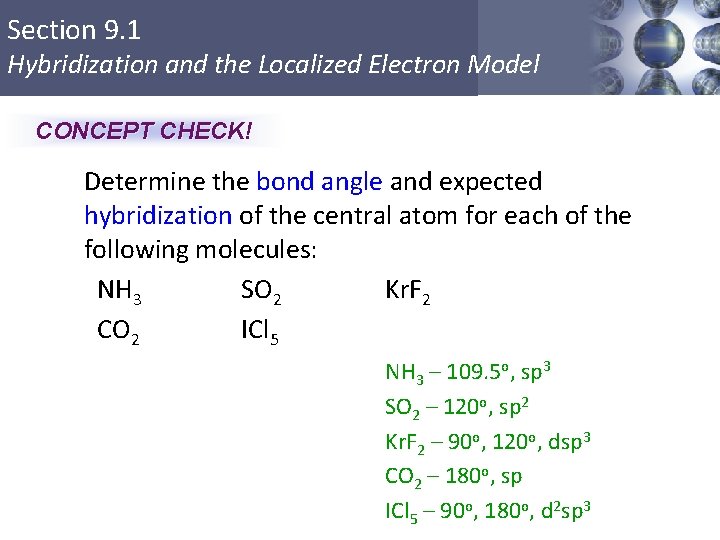
One s and one p orbital combine to form two sp hybrid orbitals. VSEPR 7 KrF2 Theory Pre-lab Lewis Structure Pre-lab Calculated by Gaussian in lab х х Hybridization of Central Atom Electron Geometry Molecular Geometry Bond Angles Dipole moment X. See the VSEPR Table and determined the hydridization of the central atom in a molecule.
The molecule is nonpolar because the two Kr-F bond dipoles cancel each other. Therefore the hybridization of Cl is sp3d2 hybridization. Alcl3 hybridization of central atom by 01122020 Condos For Rent In Bellevue Tn 37221 Skin On Fries - Aldi Pomfret Fish Price In Delhi Macys Technical Error Sg Standard 61 Maestro Vibrola Skyrim Multiple Pets Mod The Andersons Innova Organic Fertilizer.
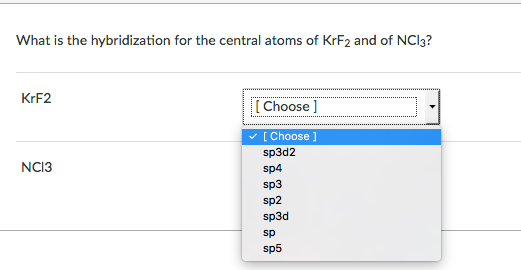
Krf2 hybridization of central atom Uncategorized krf2 hybridization of central atom. Solution for In the KrF2 molecule the central atom Kr has 2 bonding pairs and 3 nonbonding pairs of electrons. Solved Expert Answer to What is the hybridization for the central atoms of KrF2 and of NCl3.
So in that model as well the hybridization of the bromine is sp3. The molecular shape ignores the lone pairs so the VSEPR shape is square planar. For a molecule geometry and shape are the same only when there are no lone pairs of electrons around the central atom.
6 Use and Manufacturing Expand this section. BbKrF_2 The Lewis structure of KrF_2 is. 4 Related Records Expand this section.
If you look at the octet model the one I dont like because it has too many charges you will see the bromine also has four domains around it. KrF4 XeCl2 and XeBr2 are predicted to be rather unstable against molecular dissociation. 1 Structures Expand this section.
Two single bonds and two lone pairs. The central atom has 2 lone pairs and 4 bond pairs. The central atom has 3 lone pairs and 2 bond pairs.
What is the hybridization of bro2. Krypton is the central atom in this case because it is the least electronegative of the two atoms involved as it has an electronegativity of 30 and Fluorine has an electronegativity of 40. Krypton fluoride KrF2 UNII-A91DJL4OJC.
With nitrogen however there are five rather than four valence electrons to account for meaning that three of the four hybrid orbitals are half-filled and available for bonding while the fourth is fully occupied by a non-bonding pair of electrons. Hybridization is based on the electron geometry. It is a theoretical concept.
Molecular Geometry Bond Angles CI. The crystal field model is capable of reproducing all significant differences observed between the gas phase and the solid state. Thus the steric number SN is 6 and the electron geometry is octahedral.
CH 2 O 3. Just like the carbon atom in methane the central nitrogen in ammonia is sp 3-hybridized. The shape and energy of hybrid orbitals are the same.
The hybridization of orbitals on the central atom in a molecule is sp. Hybridization and Valence Bond Theory.

Krf2 Lewis Structure Hybridization Molecular Geometry And Polarity Techiescientist

Krf2 Lewis Structure Hybridization Molecular Geometry And Polarity Techiescientist

The Hybrid Orbital Set Used By The Central Atom In Chegg Com

Krf2 Lewis Structure Hybridization Molecular Geometry And Polarity Techiescientist

Krf2 Lewis Structure Hybridization Molecular Geometry And Polarity Techiescientist

What Is The Hybridization For The Central Atoms Of Chegg Com

Krf2 Lewis Structure Hybridization Molecular Geometry And Polarity Techiescientist
Hybridization Of Atomic Orbitals Ck 12 Foundation
Advanced Theories Of Covalent Bonding Valence Bond Theory
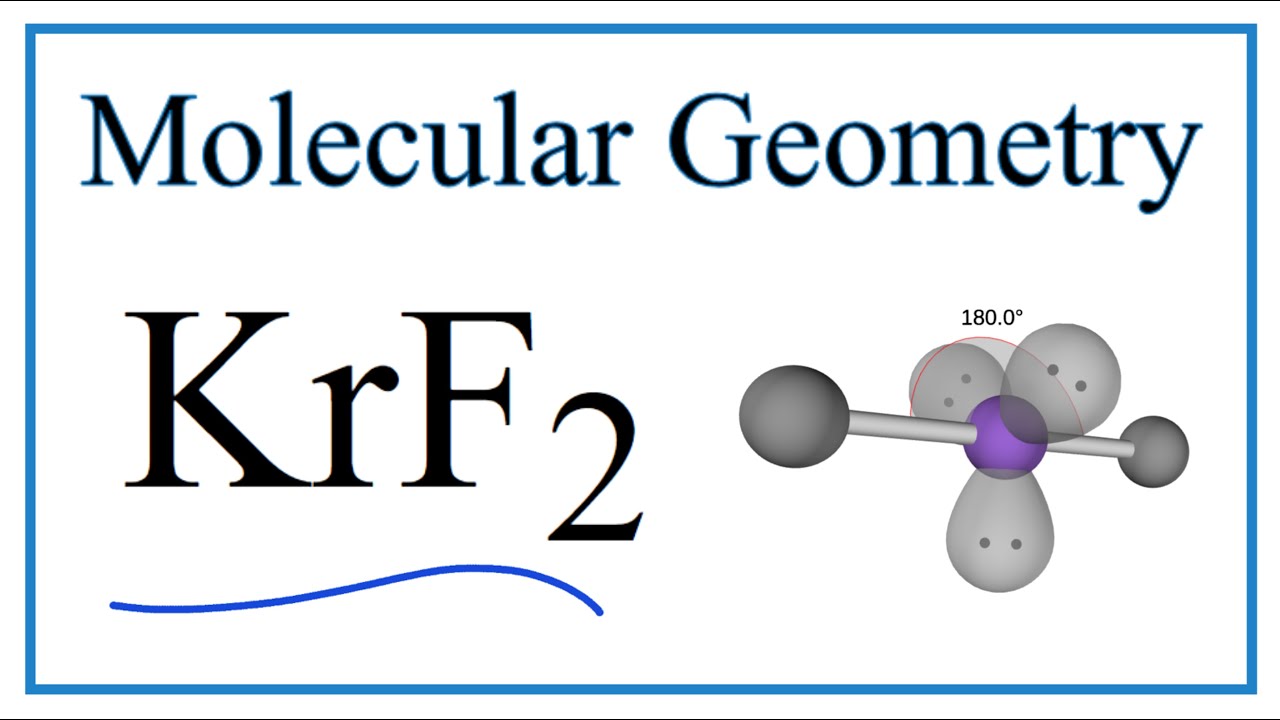
Krf2 Molecular Geometry Bond Angles And Polarity Youtube
Hybridization Of Atomic Orbitals Read User Generated Content Ck 12 Foundation

Ap Chemistry Ch 9 Covalent Bonding Orbitals Ppt Download

Krf2 Lewis Structure Hybridization Molecular Geometry And Polarity Techiescientist
Localized E Model And Hybrid Orbitals Sigma And Pi Bonds Ppt Video Online Download
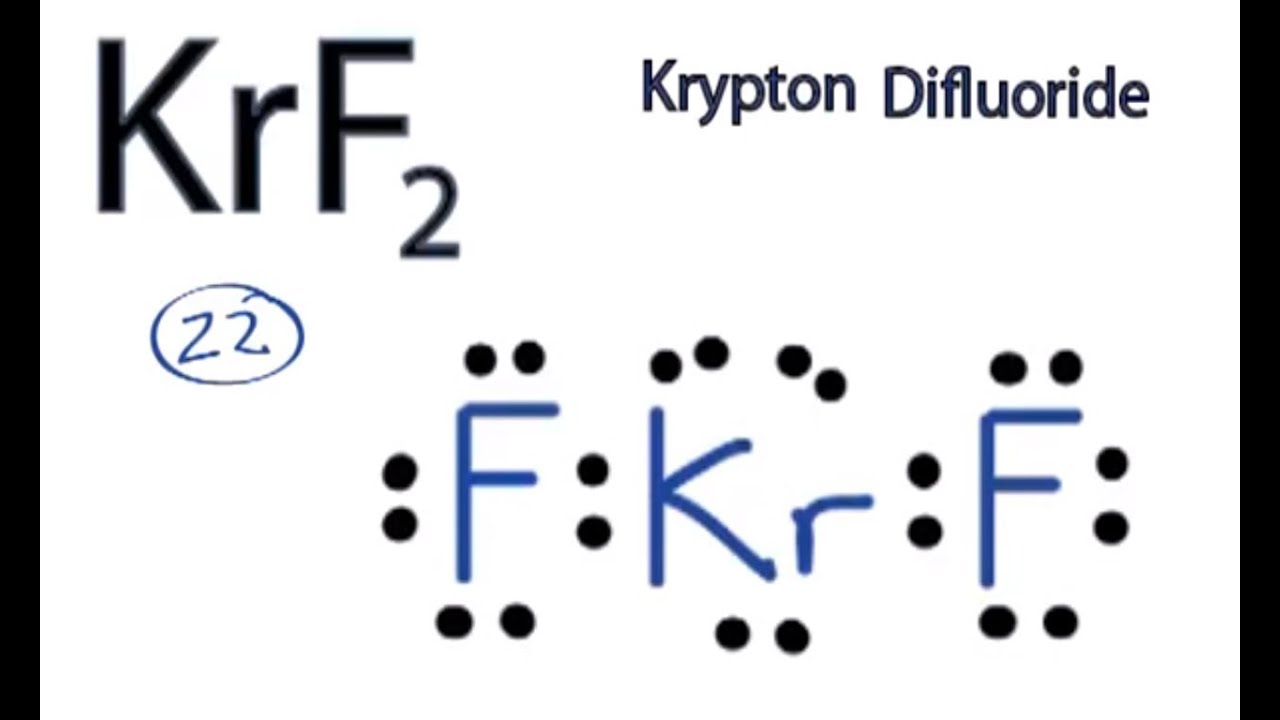
Krf2 Molecular Geometry Bond Angles And Polarity Youtube



0 Response to "Krf2 Hybridization Of Central Atom"
Posting Komentar