Krf2 Octet Rule
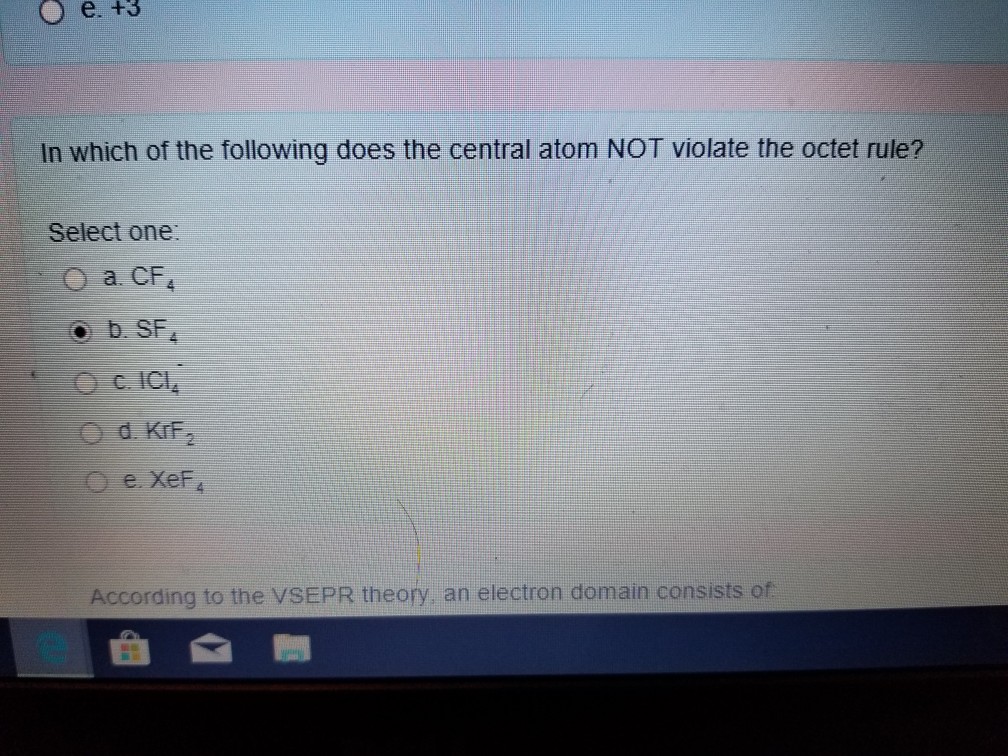
The octet rule states that elements tend to prefer to have eight electrons in their valence shell. The Central Atom In A SF4 B KrF2 C CF D XeF4 Does Net Violate The Octet Rule.

Krf2 Lewis Structure Hybridization Molecular Geometry And Polarity Techiescientist
C PH3 i SF Discussion.

Krf2 octet rule. A NF3 B SO2 C BC13 D CF4 E SO32. CF4 is correct for The central atom in _____ does not violate the octet rule. The Electron Configuration Of The Sulfide Ion S2-is A İNel3s2 B Ne3s23pl C Ne3823p4 D Nel3p2 E INej3s23p6 14.
Xenon and krypton also combine with oxygen and fluorine to form compounds such as XeF2 KrF2 XeOF2 etc. Thankyou for using answerout. The central atom in _____ does not violate the octet rule.
We hope you get all your answers here. Each atom gets 8 electrons except H which gets two. As per octet rule atoms can combine either by transfer of valence electrons from one atom to another or by sharing of valence electrons in order to have an octet in their valence shell.
The maximum number of valence outer shell electrons is 8. If a species is an ion subtract one electron for each unit of positive charge. Complete octets around all atoms using unshared pairs 4.
Draw Lewis structures that obey the octet rule for the following species. In which of the molecules below is the carbon-carbon distance the. Assign the formal charge to each central atom.
21 The reaction below is used to produce methanol. Write the Lewis structures of the following molecules. The shape of the molecules does not account for this theory.
If you are having trouble with Chemistry Organic Physics Calculus or Statistics we got your back. A SF4 B KrF2 C CF4 D XeF4 E ICl4-C CF4. Show all resonance forms.
Does krf2 violate the octet rule. The central atom in SF₄ does not violate the octet rule. Our videos will help you understand concepts solve your homework and do great on your exams.
Ar 18 3995 He Ne Ar Kr Xe Rn Og 18p 2𝑒 8𝑒 8𝑒 valence electrons 8 Note. а ВеНz g SeF4 b H2S h KrFs d CF4 i PF5 e IF3 k KrF2 f XEF4 1 BCI3. Which Of The.
Which Of The Following Lewis Structures Would Be An Incomplete Octer. The rule of the octet is evidently based on the chemical inertness of the noble gases. The central atom in CF₄ does not violate the octet rule.
However some noble gasses eg. Using hypercon-jugation draw the Lewis structures for KrF2 that obey the octet rule. Chemical Principles 8th Edition Edit edition Solutions for Chapter 13 Problem 133CP.
As per octet rule atoms can combine either by transfer of valence electrons from one atom to another or by sharing of valence electrons in order to have an octet in their valence shell. If you have any special questions you can comment to. A 3 NF B 2 BeH C 2 SO D CF 4 E SO 3 2- Answer.
Predict the molecular structure of KrF2. 87 92 Why dont we draw double bonds between the Be atom and the Cl atoms in 2 BeCl. The 3rd shell is not actually full yet but the next 2 electrons will orbit in the 4th shell.
The central atom in _____ obeys the octet rule. The central atom in SF₄ does not violate the octet rule. Although they are few some stable compounds have an odd number of electrons in their valence shells.
AKrF2 BXeF4 CCF4 DICl4- ESF4 SHORT ANSWER. The Lewis structure of N2H2 shows _____ Each nitrogen has one nonbonding electron pair. If an anion add one electron for.
Each atom brings a number of valence electrons to a molecule equal to its group designation eg C in 4A brings 4 O in 6A brings 6. Our videos prepare you to succeed in your college classes. Students also viewed these Organic Chemistry questions.
Let us help you simplify your studying. ICI4-The Correct Answer is. Perspective Drawing Of Krf2 Best Way Drawing Perspective Drawing Of Krf2.
The most common exceptions to the octet rule are the so-called hypervalent compounds. 20 The central atom in _____ does not violate the octet rule. Which molecules are expected to violate the octet rule.
Using hyperconjugation draw the Lewis structures for KrF2 that obey the octet rule. Show all resonance forms. 91 A valid Lewis structure of _____ cannot be drawn without violating the octet rule.
The central atom in _____ does not violate the octet rule. Write the word or phrase that best completes each statement or answers the question. CO g 2 H2 g CH3OH l DHrxn.
With the octet rule things are simple.
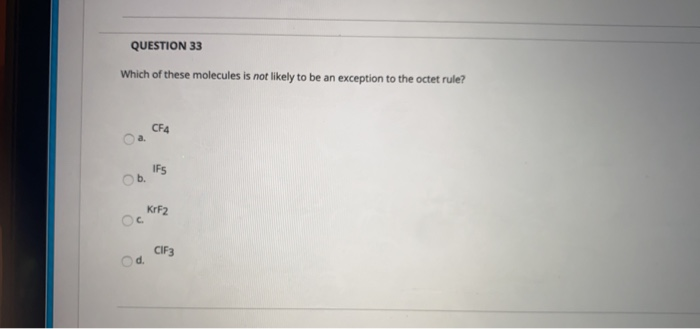
Question 33 Which Of These Molecules Is Not Likely To Chegg Com
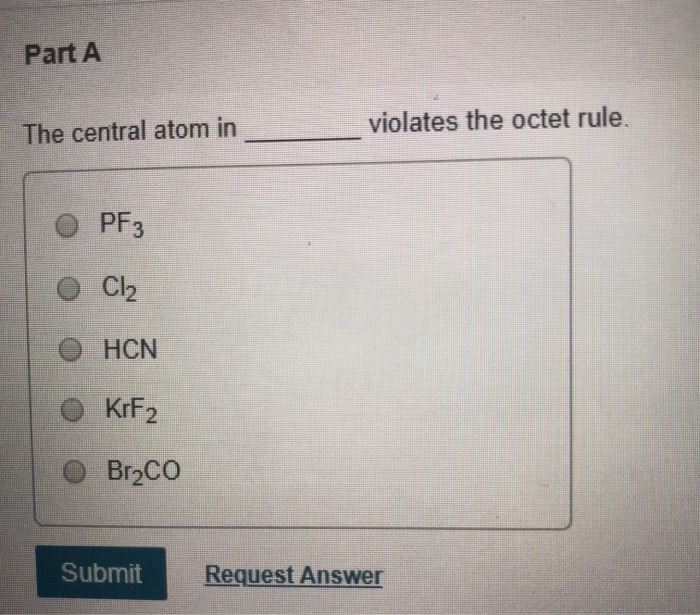
Part A The Central Atom In Violates The Octet Rule Chegg Com
E 3 In Which Of The Following Does The Central Atom Chegg Com

Among Xeof2 Scl2 Krf2 Nf3 No How Many Of Them Violate Octet Chemistry Chemical Bonding 12892963 Meritnation Com
Which Of These Compounds Violate The Octet Rule Chegg Com

Draw The Lewis Structure For Krf2 The Cen Clutch Prep

What Is The Lewis Dot Structure Krf2 How Is It Determined Quora

Krf2 Lewis Structure Hybridization Molecular Geometry And Polarity Techiescientist
Which Of These Compounds Violate The Octet Rule Chegg Com
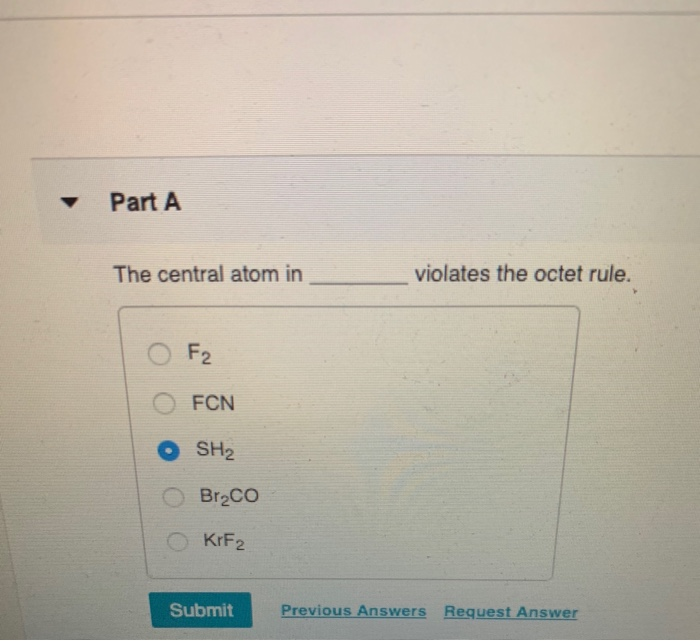
The Central Atom In Violates The Octet Rule Chegg Com

Krf2 Lewis Structure Hybridization Molecular Geometry And Polarity Techiescientist
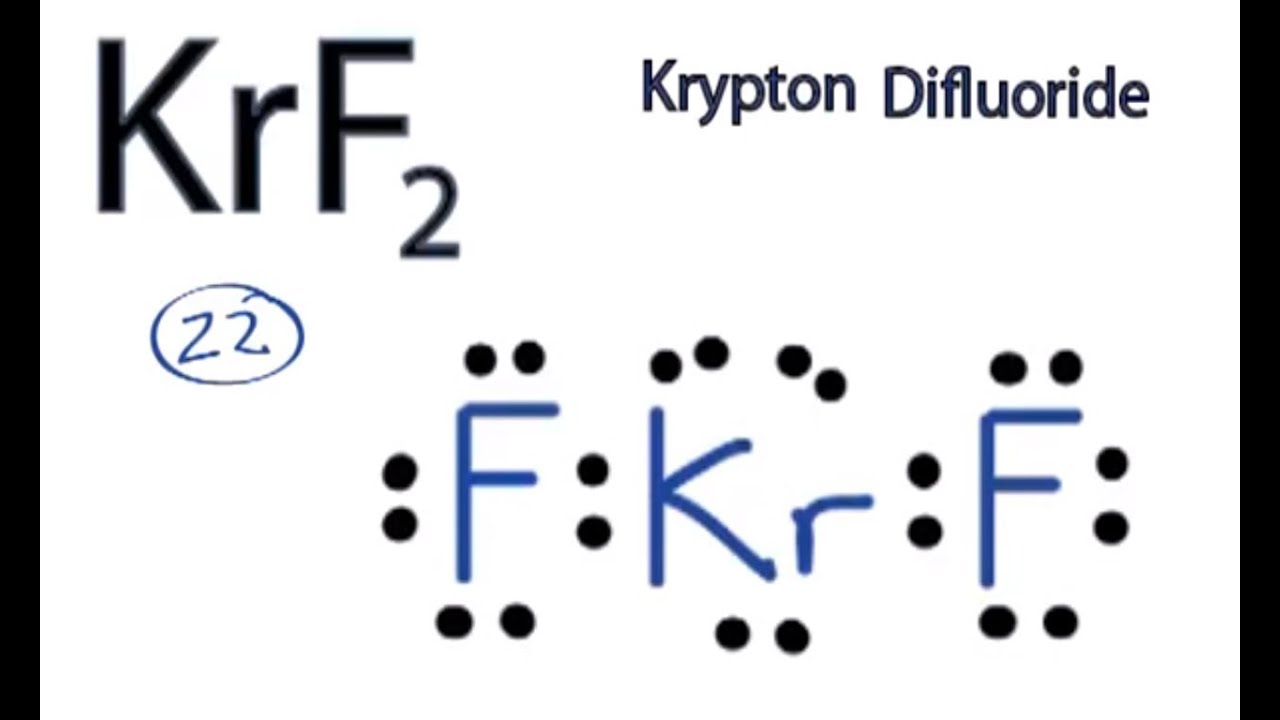
Krf2 Lewis Structure How To Draw The Lewis Structure For Krf2 Krypton Difluoride Youtube

The Central Atom In Does Not Violate The Octet Rule Chegg Com
Which Of These Compounds Violate The Octet Rule Chegg Com

Draw The Lewis Structure For Krf2 The Cen Clutch Prep

Krf2 Lewis Structure Hybridization Molecular Geometry And Polarity Techiescientist
22 The Central Atom In Does Not Violate The Octet Chegg Com





0 Response to "Krf2 Octet Rule"
Posting Komentar